Exhibit 99.1

corporate presentation 2017 Symbol: BSGM

Disclaimer Symbol:BSGM * This presentation contains forward-looking statements including statements that address activities, events or developments that BioSig expects, believes or anticipates will or may occur in the future, such as predictions of financial performance, approvals and launches by BioSig of new products, market acceptance of BioSig’s products, market and procedure projections, financing plans, and related documents. Forward-looking statements are based on BioSig’s experience and perception of current conditions, trends, expected future developments and other factors it believes are appropriate under the circumstances and are subject to numerous risks and uncertainties, many of which are beyond BioSig’s control. These risks and uncertainties include the timing of approvals for BioSig products, rate and degree of market acceptance of products, BioSig’s ability to develop and market new and enhanced products, the timing of and ability to obtain and maintain regulatory clearances and approvals for its products and the impact of failure to obtain such clearances and approvals on its ability to promote its products and train doctors and operators in the use of its products, the timing of and ability to obtain reimbursement if required of procedures utilizing BioSig’s products and the potential impact of current healthcare reform initiatives thereon, competition from existing and new products and procedures or BioSig’s ability to effectively react to other risks and uncertainties described from time to time in BioSig’s SEC filings, such as fluctuation of financial results, reliance on third party manufacturers and suppliers, litigation or other proceedings, government regulation, negative publicity, current worldwide economic conditions and share price volatility. BioSig does not guarantee any forward-looking statements, and actual results may differ materially from those projected. Unless required by law, BioSig undertakes no obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.
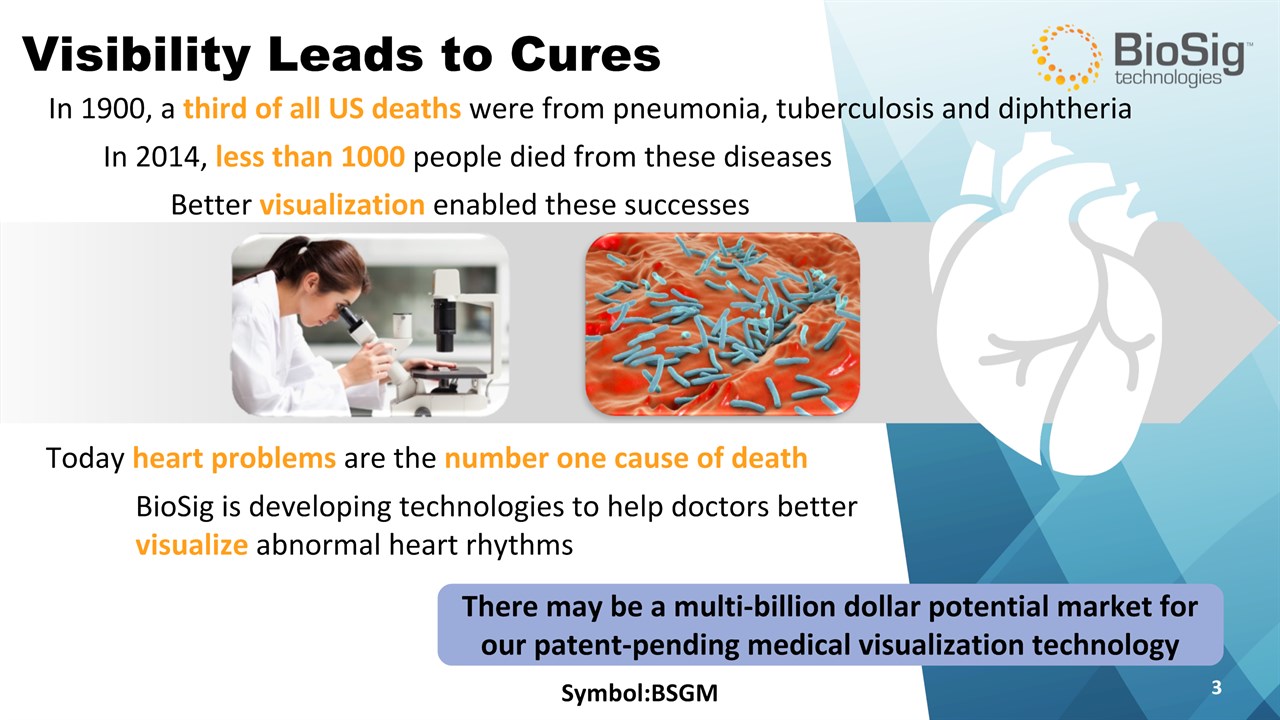
Symbol:BSGM * Visibility Leads to Cures In 1900, a third of all US deaths were from pneumonia, tuberculosis and diphtheria In 2014, less than 1000 people died from these diseases Today heart problems are the number one cause of death Better visualization enabled these successes BioSig is developing technologies to help doctors better visualize abnormal heart rhythms There may be a multi-billion dollar potential market for our patent-pending medical visualization technology
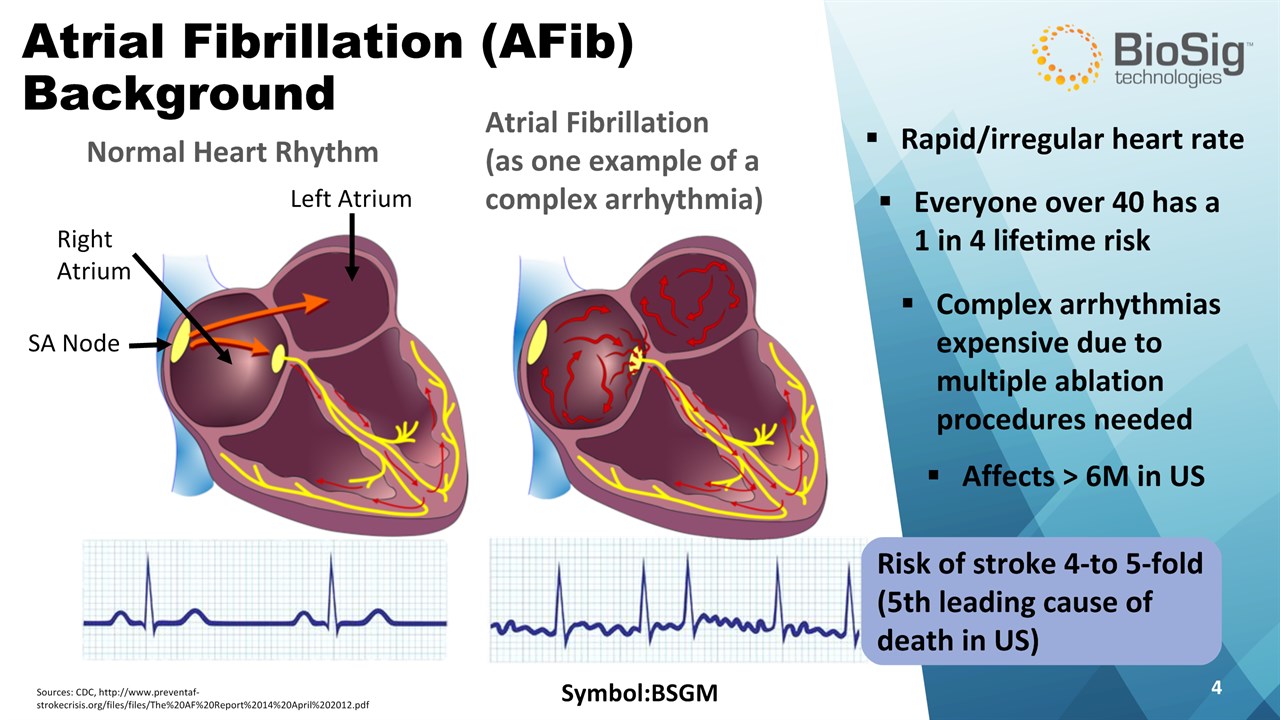
Risk of stroke 4-to 5-fold(5th leading cause of death in US) Symbol:BSGM * Atrial Fibrillation (AFib)Background Normal Heart Rhythm Atrial Fibrillation (as one example of acomplex arrhythmia) Everyone over 40 has a 1 in 4 lifetime risk Affects > 6M in US Rapid/irregular heart rate Complex arrhythmias expensive due to multiple ablation procedures needed Sources: CDC, http://www.preventaf-strokecrisis.org/files/files/The%20AF%20Report%2014%20April%202012.pdf Left Atrium Right Atrium SA Node
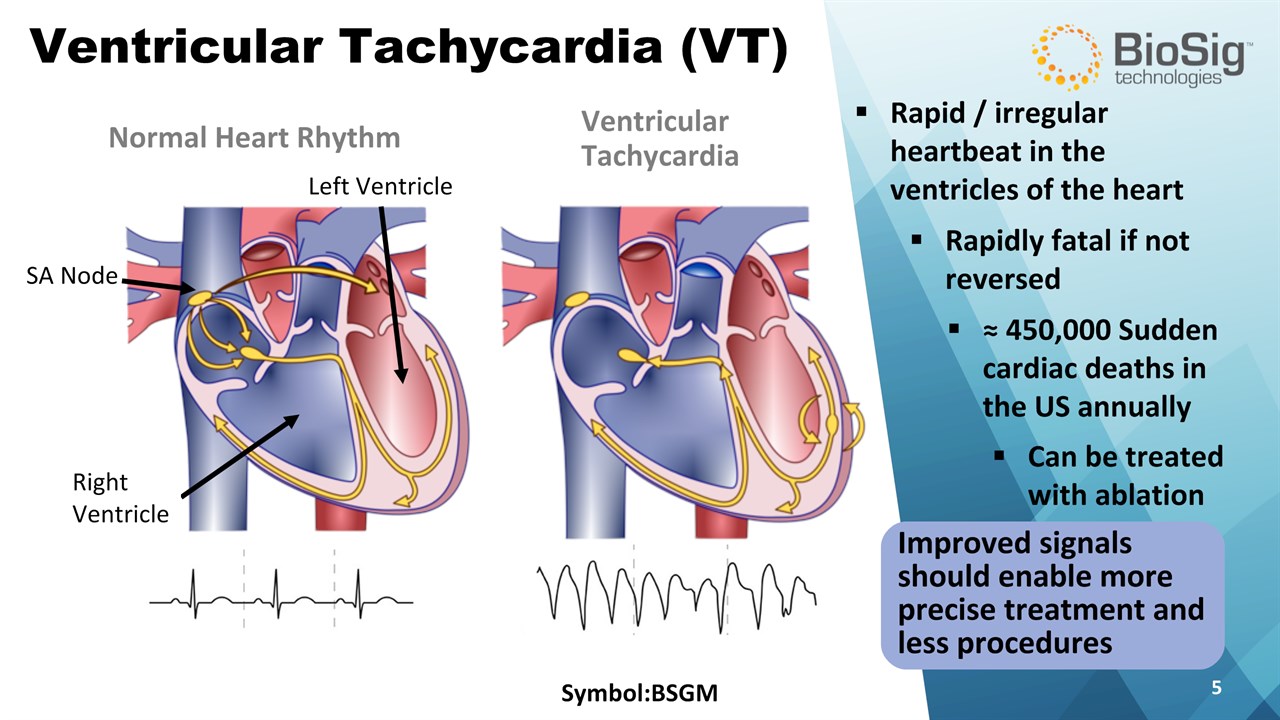
Improved signals should enable more precise treatment and less procedures Ventricular Tachycardia (VT) Symbol:BSGM * Normal Heart Rhythm VentricularTachycardia ≈ 450,000 Sudden cardiac deaths in the US annually Rapid / irregular heartbeat in the ventricles of the heart Rapidly fatal if not reversed Can be treated with ablation Left Ventricle SA Node Right Ventricle
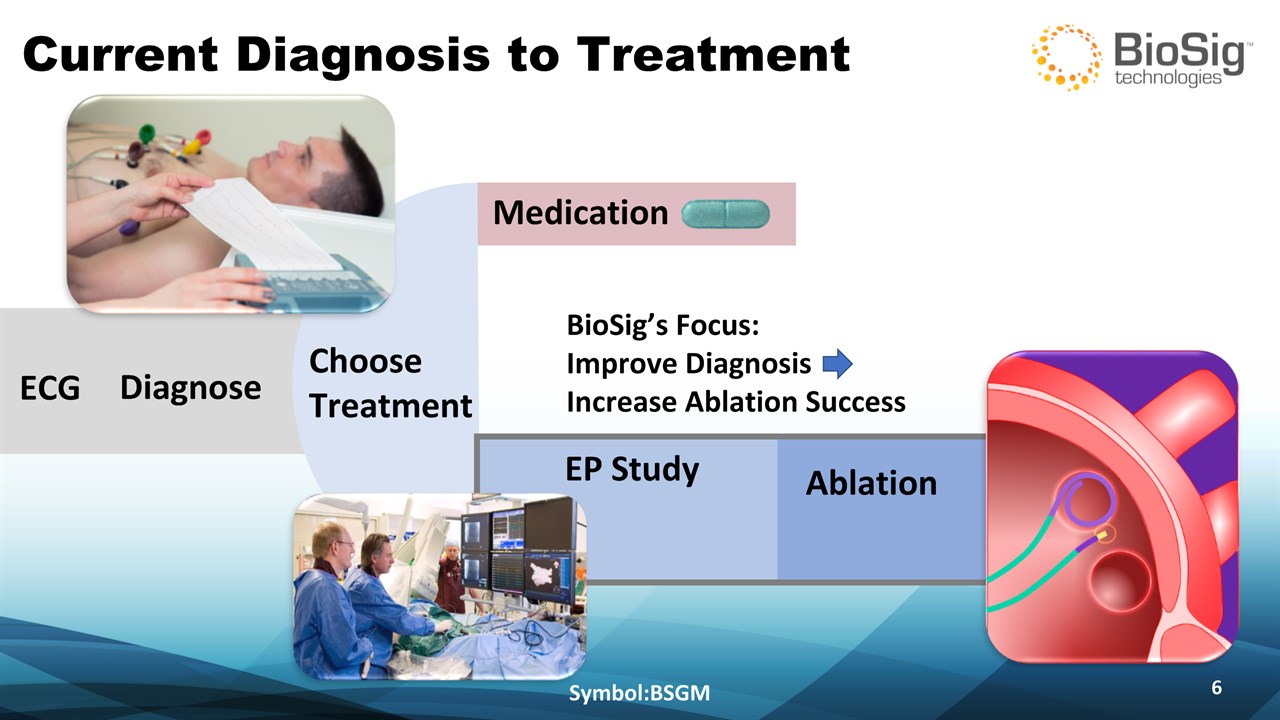
Current Diagnosis to Treatment Symbol:BSGM * ECG Medication EP Study Ablation Diagnose ChooseTreatment BioSig’s Focus: Improve Diagnosis Increase Ablation Success
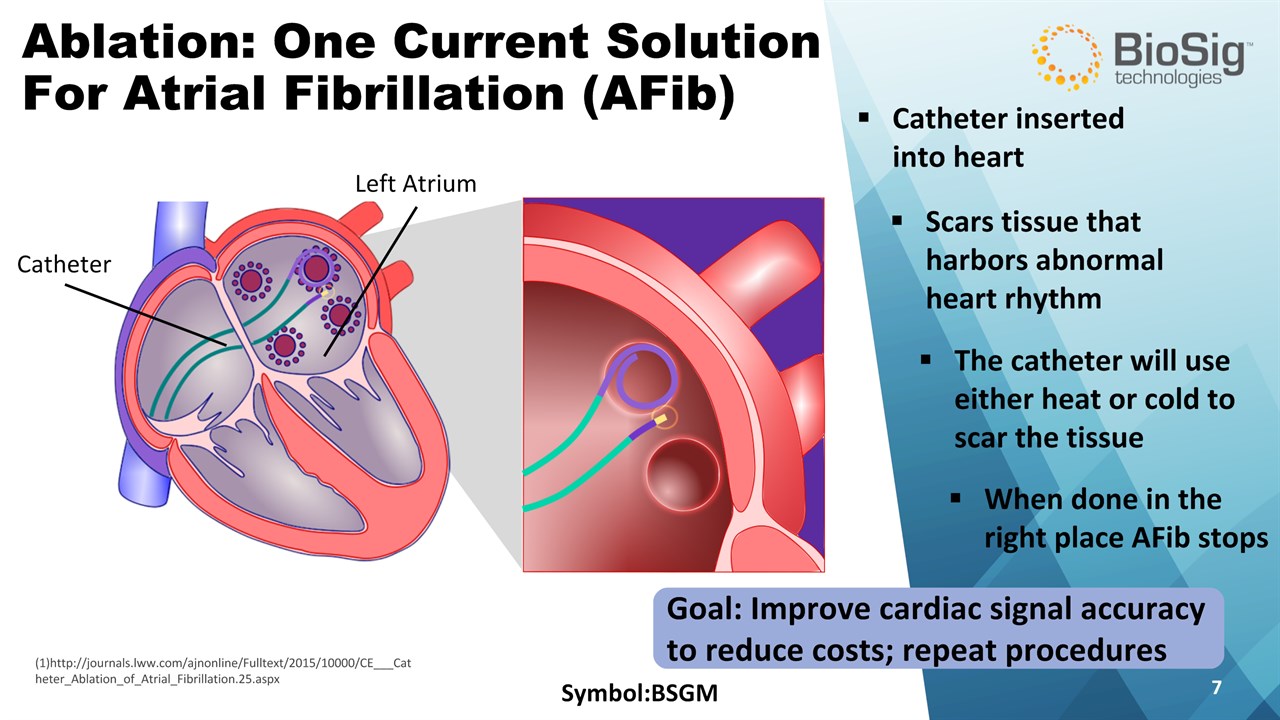
Goal: Improve cardiac signal accuracy to reduce costs; repeat procedures Symbol:BSGM * Ablation: One Current SolutionFor Atrial Fibrillation (AFib) Catheter inserted into heart Scars tissue that harbors abnormal heart rhythm Left Atrium Catheter (1)http://journals.lww.com/ajnonline/Fulltext/2015/10000/CE___Catheter_Ablation_of_Atrial_Fibrillation.25.aspx The catheter will use either heat or cold to scar the tissue When done in the right place AFib stops
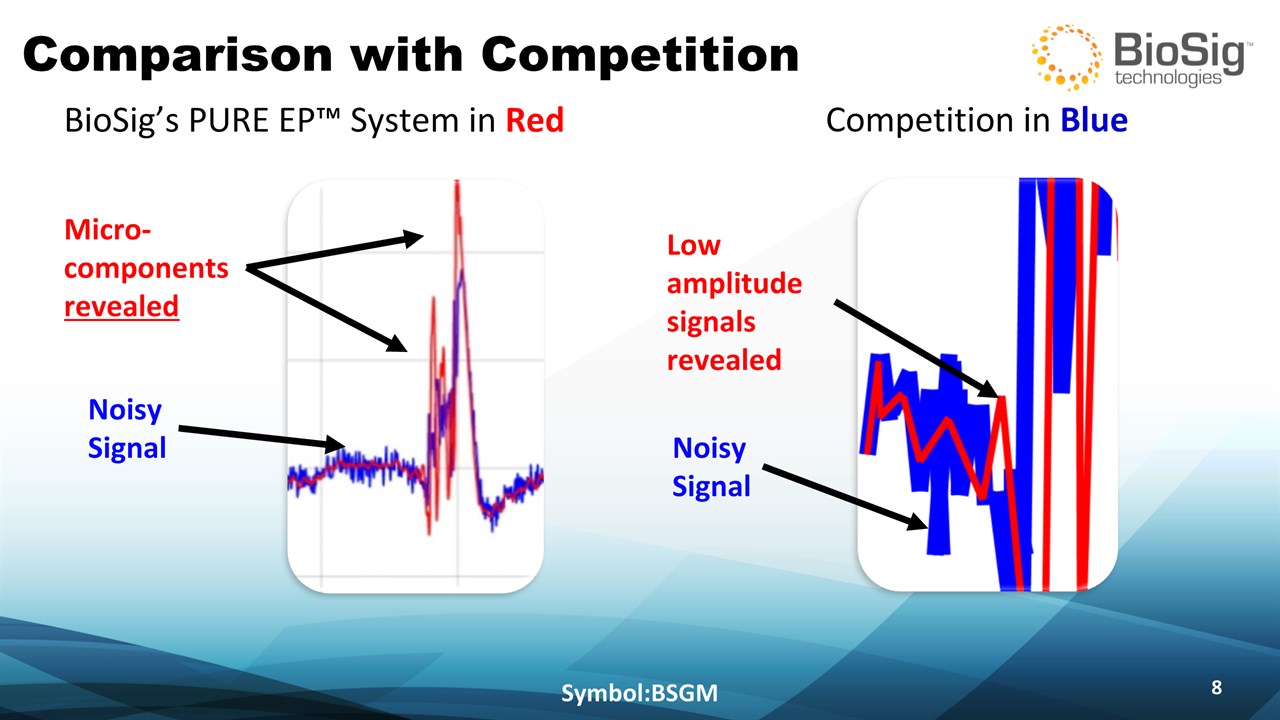
Comparison with Competition Symbol:BSGM * Micro-componentsrevealed Competition in Blue BioSig’s PURE EP™ System in Red Noisy Signal Noisy Signal Low amplitude signals revealed
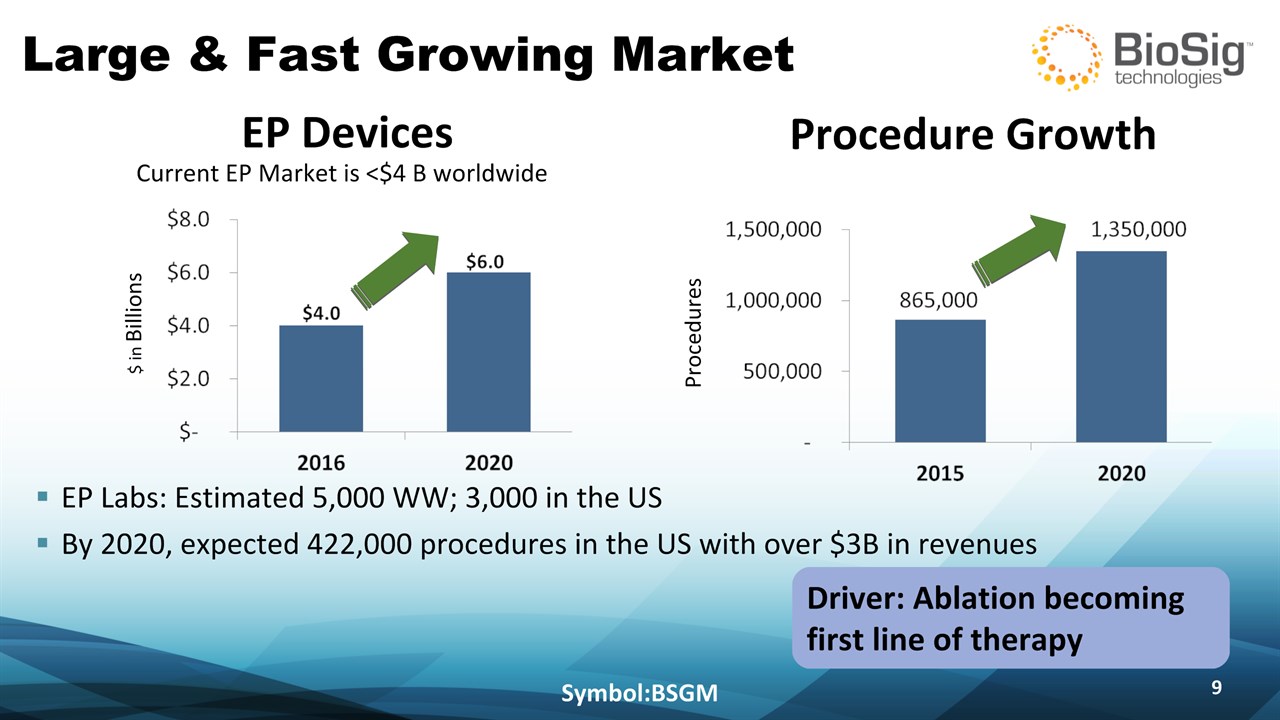
Driver: Ablation becoming first line of therapy Large & Fast Growing Market Symbol:BSGM * EP Labs: Estimated 5,000 WW; 3,000 in the USBy 2020, expected 422,000 procedures in the US with over $3B in revenues EP Devices Current EP Market is <$4 B worldwide $ in Billions Procedure Growth Procedures
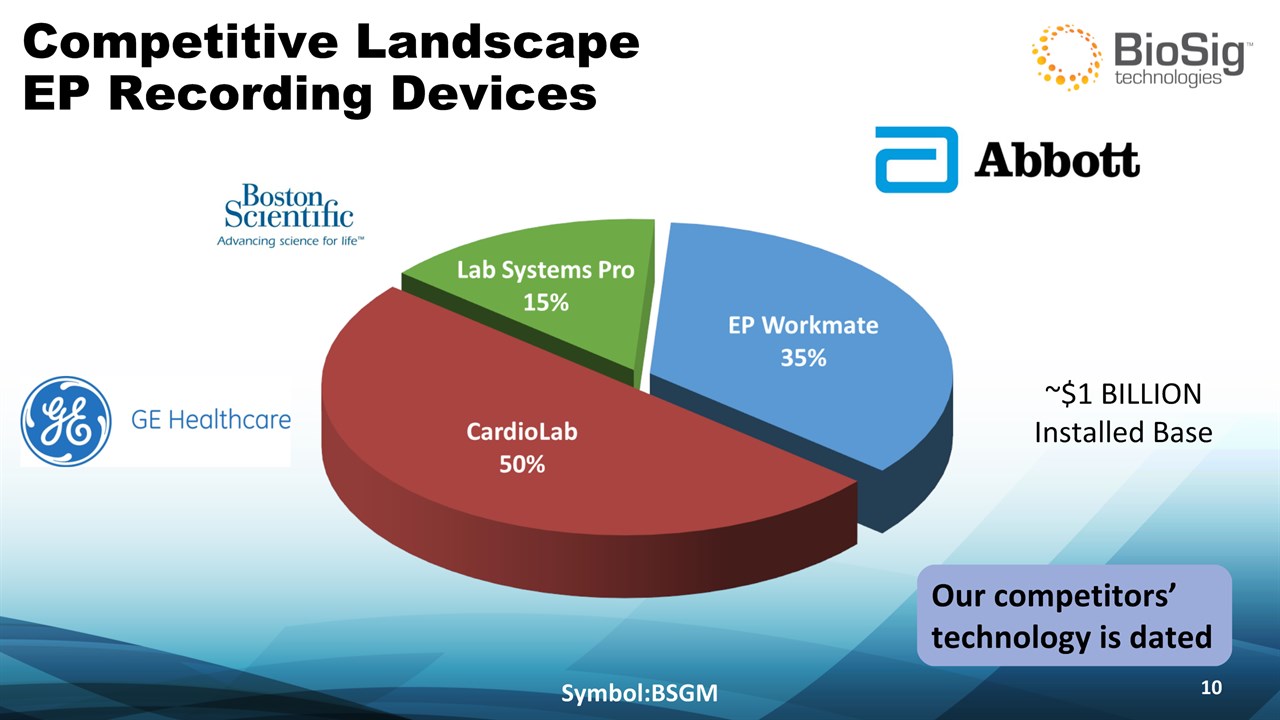
Competitive Landscape EP Recording Devices Symbol:BSGM * ~$1 BILLION Installed Base Our competitors’ technology is dated
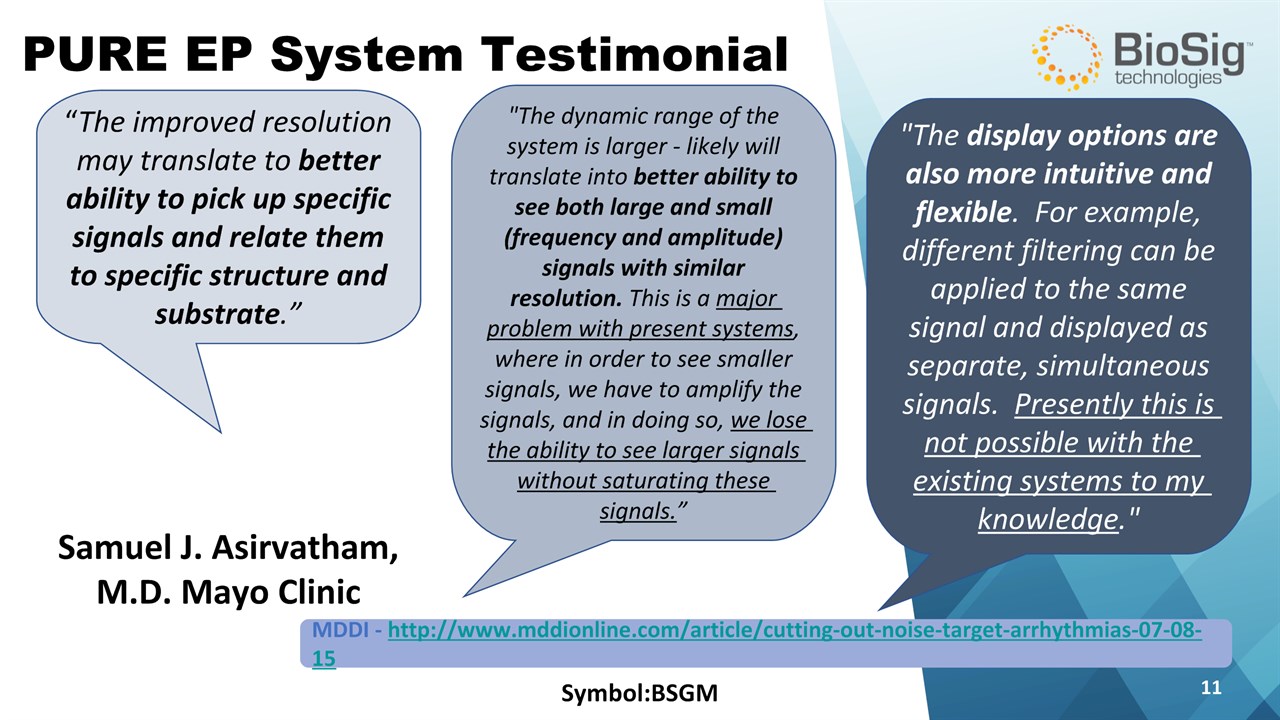
Symbol:BSGM * PURE EP System Testimonial "The dynamic range of the system is larger - likely will translate into better ability to see both large and small (frequency and amplitude) signals with similar resolution. This is a major problem with present systems, where in order to see smaller signals, we have to amplify the signals, and in doing so, we lose the ability to see larger signals without saturating these signals.” “The improved resolution may translate to better ability to pick up specific signals and relate them to specific structure and substrate.” "The display options are also more intuitive and flexible. For example, different filtering can be applied to the same signal and displayed as separate, simultaneous signals. Presently this is not possible with the existing systems to my knowledge." Samuel J. Asirvatham, M.D. Mayo Clinic MDDI - http://www.mddionline.com/article/cutting-out-noise-target-arrhythmias-07-08-15
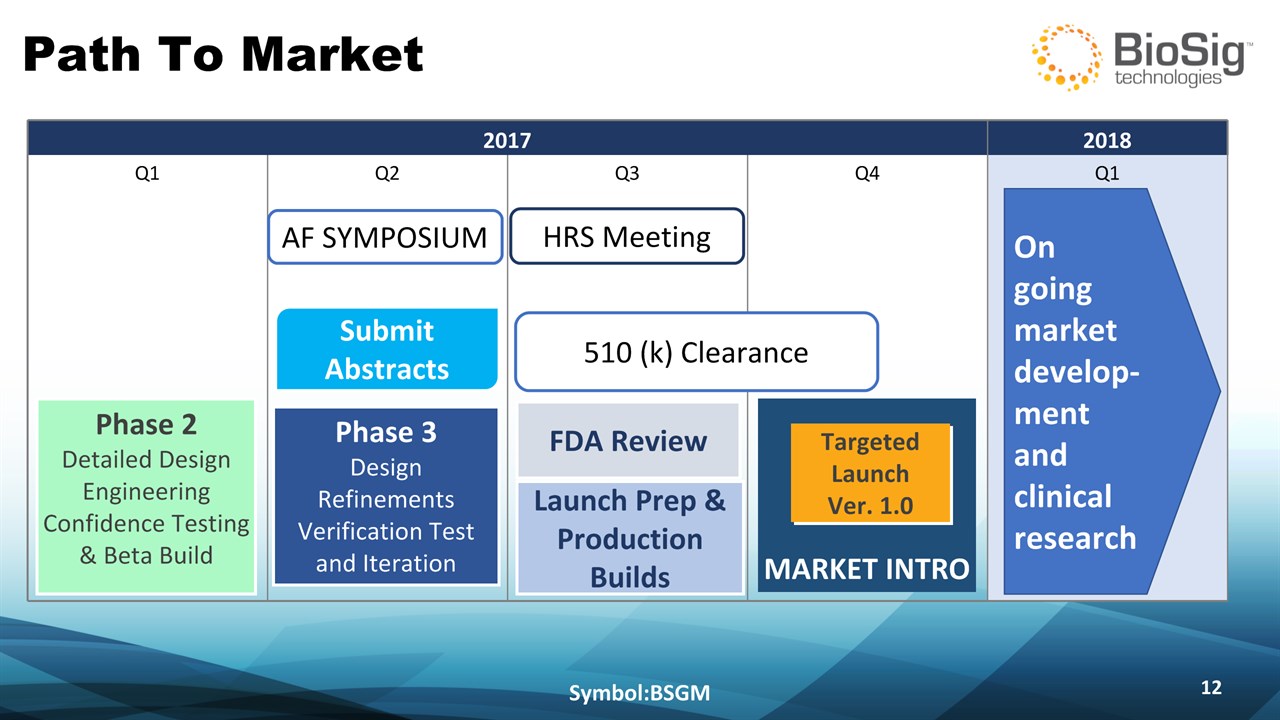
Path To Market Symbol:BSGM * 2017 2017 2017 2017 2018 Q1 Q2 Q3 Q4 Q1 FDA Review Launch Prep & Production Builds MARKET INTRO Targeted Launch Ver. 1.0 AF SYMPOSIUM HRS Meeting Submit Abstracts 510 (k) Clearance Phase 3Design Refinements Verification Test and Iteration Phase 2Detailed DesignEngineering Confidence Testing & Beta Build On going market develop-mentand clinical research
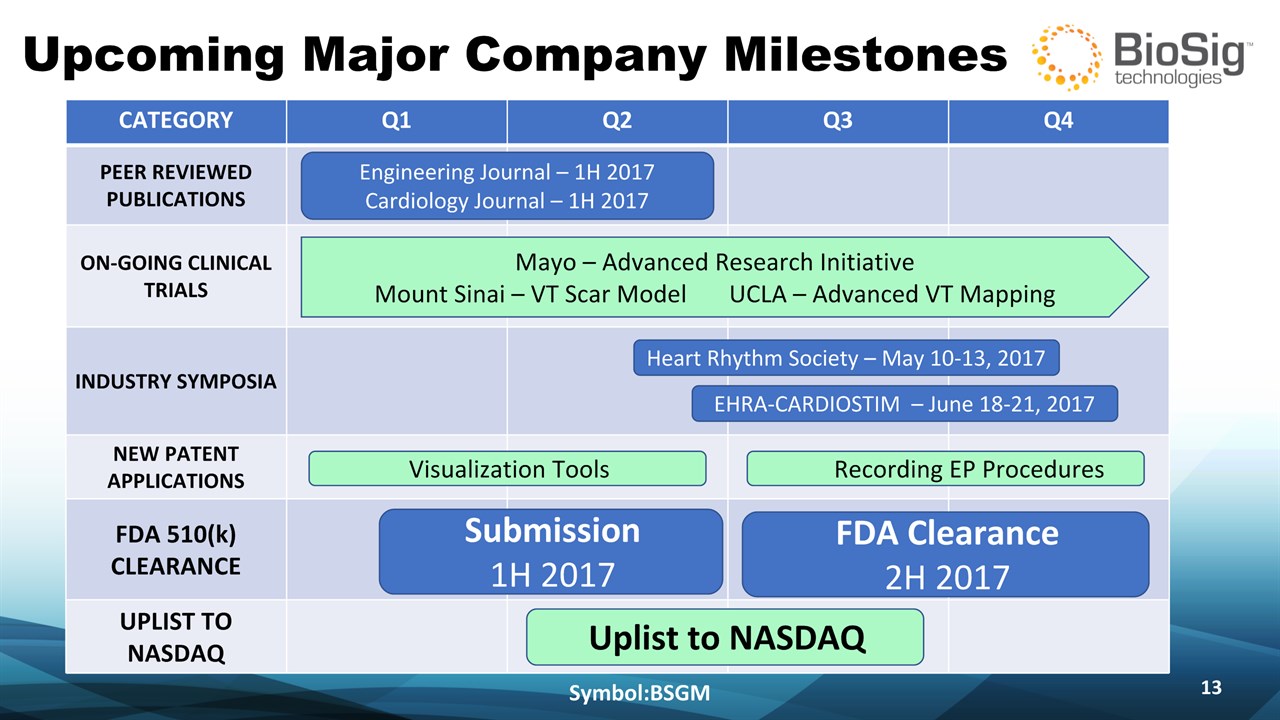
Upcoming Major Company Milestones Symbol:BSGM * CATEGORY Q1 Q2 Q3 Q4 PEER REVIEWED PUBLICATIONS ON-GOING CLINICAL TRIALS INDUSTRY SYMPOSIA NEW PATENT APPLICATIONS FDA 510(k) CLEARANCE UPLIST TO NASDAQ Engineering Journal – 1H 2017Cardiology Journal – 1H 2017 Mayo – Advanced Research InitiativeMount Sinai – VT Scar Model UCLA – Advanced VT Mapping Heart Rhythm Society – May 10-13, 2017 EHRA-CARDIOSTIM – June 18-21, 2017 Submission 1H 2017 Uplist to NASDAQ Visualization Tools Recording EP Procedures FDA Clearance 2H 2017

Symbol:BSGM * Board of Directors Kenneth L. Londoner, MBA Founder, Executive Chairman, Director; Endicott Management Partners, J & W Seligman & Co Gregory D. Cash President, CEO and Director: U.S. Surgical, Boston Scientific, Medtronic Roy T. Tanaka Director; Former CEO of BioSense Webster, Johnson & Johnson; Volcano Corp, VytronUS, Coherex Medical Seth H. Z. Fischer Director; Current CEO & Dir: Vivus, Inc; Former WW Chairman: Johnson & Johnson, Cardiovascular Patrick J. Gallagher, MBA Director; Managing Director Laidlaw & Co.; Kinex Pharma; Founder BDR Research Group, Kidder Peabody Jeffrey F. O’Donnell, Sr. Director; CEO, Chair: Trice Medical; Chair: Mela Sciences; Founder: Embrella Cardiovascular Jerome B. Zeldis, MD, PhD Director; CEO of Celgene Global Health & Chief Medical Officer of Celgene Corporation; Chairman: Alliqua David Weild IV, MBA Director; Founder & CEO: Weild & Co.; Vice Chairman: NASDAQ; Head of Corporate Finance Prudential Donald E. Foley Director; CEO & Chair: Wilmington Trust; Sr VP, Treas, & Dir: ITT Corp; Asst Treas: Int’l Paper Co.

Proven Management Team Symbol:BSGM * Kenneth L. Londoner, MBA Founder, Executive Chairman, Director; Endicott Management Partners, LLC, J & W Seligman & Co Gregory D. Cash President, CEO and Director; Pres: Argent International, NeuroTherm, Heartsine Tech, Vasomedical, Datascope, Eminent Tech; Mgmt: U.S. Surgical, Boston Scientific, Medtronic Steve Chaussy, CPA CFO; Liberski Inc, Anna & Co, Penske Automotive, Ford Hogg and Cobbe Jay O. Millerhagen, MS, MBA VP Clinical Research; VP Clinical/Mkt Dev: RESPICARDIA, Inc.; VP/Sr Dir Clinical: St Jude Medical; Dir New Product Planning, Brady Mktg, Heart Failure R&D/Mktg, Bus Alliance Mktg with J&J, GE Healthcare: Boston Scientific Brian McLaughlin VP Corporate Finance, Investor Relations; President & COO: Ridgeback Capital; Head of Equity Trading: Sigma Capital; Trader: SAC Capital & JP Morgan & Co.
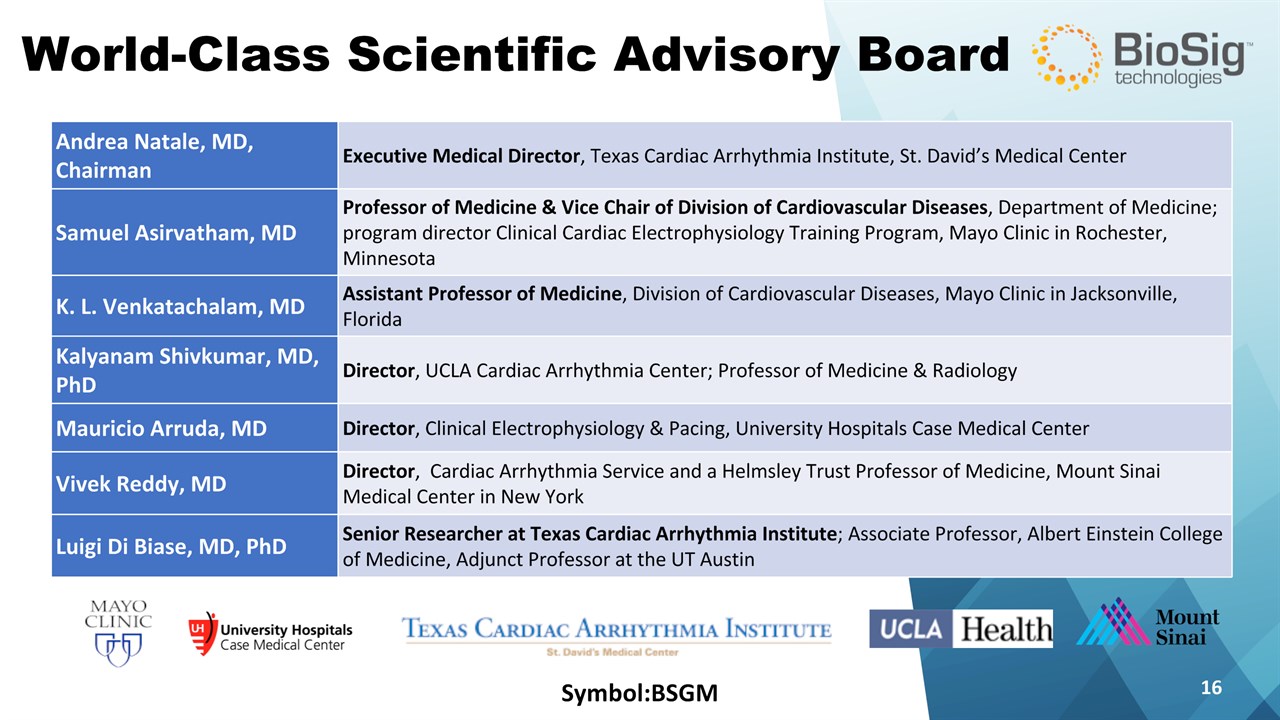
Symbol:BSGM * World-Class Scientific Advisory Board Andrea Natale, MD, Chairman Executive Medical Director, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center Samuel Asirvatham, MD Professor of Medicine & Vice Chair of Division of Cardiovascular Diseases, Department of Medicine; program director Clinical Cardiac Electrophysiology Training Program, Mayo Clinic in Rochester, Minnesota K. L. Venkatachalam, MD Assistant Professor of Medicine, Division of Cardiovascular Diseases, Mayo Clinic in Jacksonville, Florida Kalyanam Shivkumar, MD, PhD Director, UCLA Cardiac Arrhythmia Center; Professor of Medicine & Radiology Mauricio Arruda, MD Director, Clinical Electrophysiology & Pacing, University Hospitals Case Medical Center Vivek Reddy, MD Director, Cardiac Arrhythmia Service and a Helmsley Trust Professor of Medicine, Mount Sinai Medical Center in New York Luigi Di Biase, MD, PhD Senior Researcher at Texas Cardiac Arrhythmia Institute; Associate Professor, Albert Einstein College of Medicine, Adjunct Professor at the UT Austin

Presentations and Manuscripts from Pre-Clinical Studies Symbol:BSGM * Enhanced Electrophysiology Recording Improves Signal Acquisition and Differentiation Presented by team from Mayo Clinic at the 13th Annual International Dead Sea Symposium (IDSS) in Tel-Aviv and available online, March 2016 – Peer ReviewedEnhanced Electrophysiology Recording System Presented at 38th Annual International Conference EMBC 2016, August 2016 – Peer ReviewedNovel electrophysiology signal recording system enables specific visualization of the Purkinje network and other high frequency signals JACC Clinical Electrophysiology, Volume 2, Issue 7, Page 850, December 2016 – Peer Reviewed

PURE EP – Development Path Collaborating with Leading Centers Symbol:BSGM * June 2011 Concept Developed with Texas Cardiac Arrhythmia Institute June 2013 PURE EP System Proof of Concept (POC) Test at UCLA September 2014 PURE EP System Prototype Test at UCLA December 2014 Visit to Mayo Clinic to plan Pre-Clinical Studies March, June &November 2015 Pre-Clinical Studies at Mayo Clinic July 2016 Commencement of Advanced Research Program at Mayo Clinic Other Collaborations Brigham And Women’s Hospital in Boston June 2016 Initial Pre-Clinical Study at Mount Sinai in NY UH Case Medical Center in Cleveland
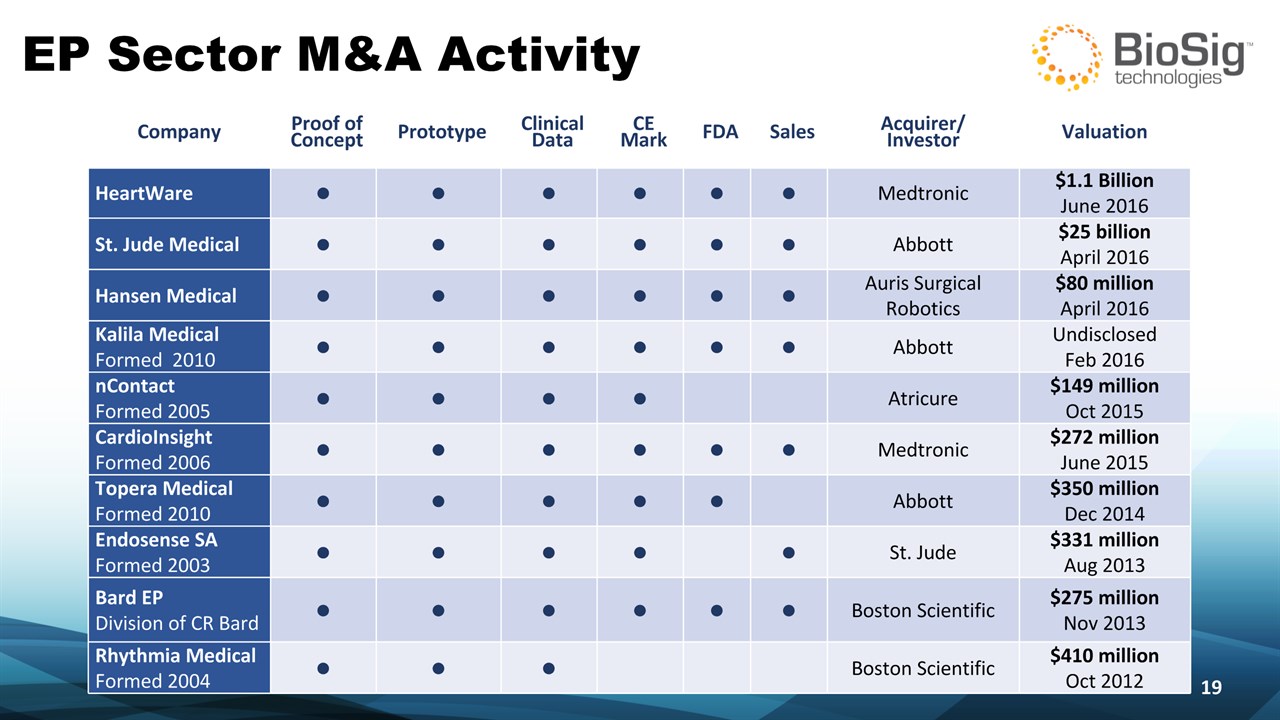
EP Sector M&A Activity * Company Proof of Concept Prototype Clinical Data CE Mark FDA Sales Acquirer/Investor Valuation HeartWare • • • • • • Medtronic $1.1 BillionJune 2016 St. Jude Medical • • • • • • Abbott $25 billionApril 2016 Hansen Medical • • • • • • Auris Surgical Robotics $80 millionApril 2016 Kalila MedicalFormed 2010 • • • • • • Abbott UndisclosedFeb 2016 nContactFormed 2005 • • • • Atricure $149 millionOct 2015 CardioInsight Formed 2006 • • • • • • Medtronic $272 millionJune 2015 Topera MedicalFormed 2010 • • • • • Abbott $350 millionDec 2014 Endosense SAFormed 2003 • • • • • St. Jude $331 millionAug 2013 Bard EPDivision of CR Bard • • • • • • Boston Scientific $275 millionNov 2013 Rhythmia MedicalFormed 2004 • • • Boston Scientific $410 millionOct 2012
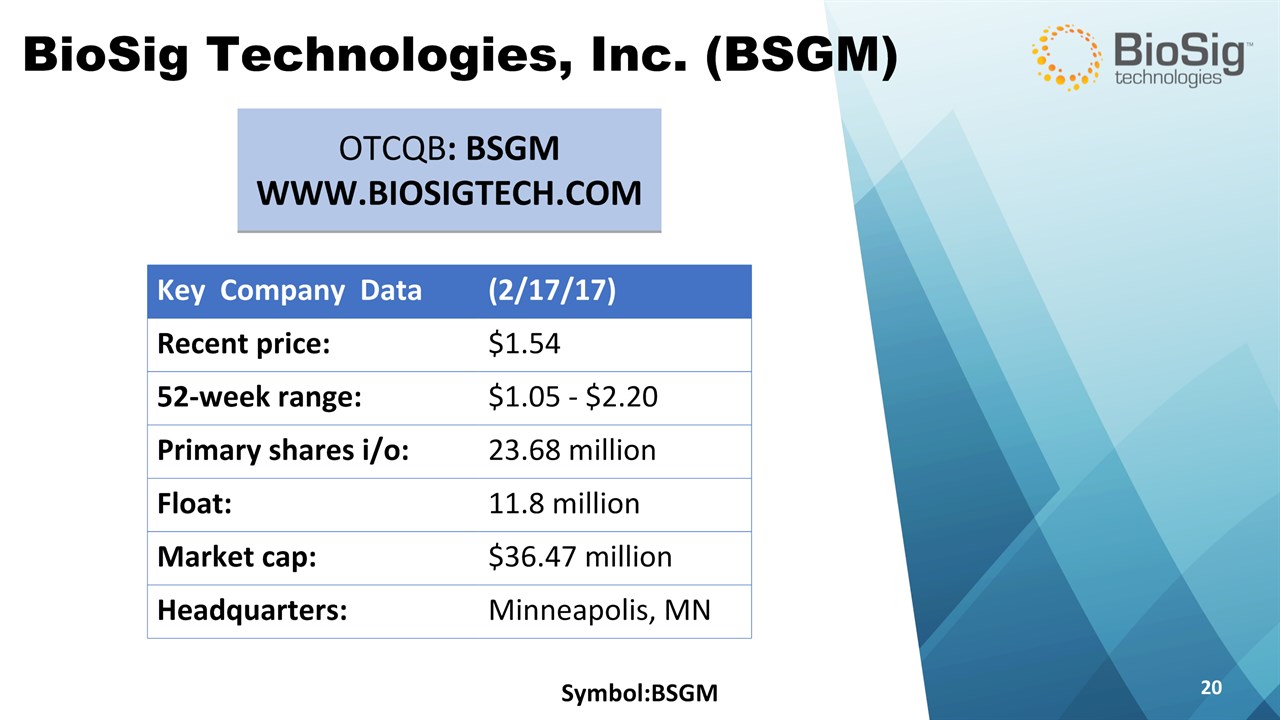
Symbol:BSGM * BioSig Technologies, Inc. (BSGM) OTCQB: BSGMWWW.BIOSIGTECH.COM Key Company Data (2/17/17) Recent price: $1.54 52-week range: $1.05 - $2.20 Primary shares i/o: 23.68 million Float: 11.8 million Market cap: $36.47 million Headquarters: Minneapolis, MN

BioSig Investment Highlights Symbol:BSGM * Large & Growing Cardiac Arrhythmia Patient Population ⇒Ablations Increasing 10%+ Annually $4B Total Addressable Market for EP Devices at 10% Growth Annually ⇒ High Demand for New Technologies World-Class Management Team, Board of Directors & Scientific Advisory Board Aggressive M&A Activity ⇒ High Growth SectorPlan to Uplist to NASDAQ in 2017FDA Submission 1H 2017 ⇒ FDA Clearance 2H 2017

Contact BioSig Symbol:BSGM * Ken LondonerFounder, Executive Chairman(203) 644-5200 klondoner@biosigtech.comGregory CashPresident & Chief Executive Officer(612) 309-4747gcash@biosigtech.comBrian McLaughlinVP, Corporate Finance & Investor Relations(917) 370-9817 bmclaughlin@biosigtech.com This document is being provided on a confidential basis by BioSig Technologies, Inc. solely for the information of those persons to whom it is transmitted. No person in any jurisdiction may treat this document as constituting either an offer to sell or solicitation of an offer to buy any securities in the Company. A prospective subscriber must rely solely on the terms of and disclosure of information including important information regarding risks and conflicts of interest contained in the Company's final offering memorandum and related documents, the only basis on which subscriptions may be made.